Liver Dysfunction and Cancer Development
Liver cancer is the fourth most common cause of cancer-related death worldwide. Hepatocellular carcinoma (HCC) accounts for 90% of primary liver cancers, with a 5-year survival rate of nearly 15%.
Obesity is associated with cellular metabolic dysfunction and a state of chronic low-grade inflammation, whereas the progression from obesity and Metabolic Dysfunction–Associated Liver Disease (MASLD) to Metabolic dysfunction-associated steatohepatitis (MASH) and HCC involves multiple insults as well as contributions from genetic modifiers and environmental factors. The mechanisms that cause HCC in some obese patients and not others remain largely unclear.
Liver cancer is refractory to nearly all currently available anticancer therapies, and there is no current method for predicting the risk of HCC development in obesity.
Group Leader, Esteban Gurzov, Ph.D.
Research Interests
Molecular mechanisms of obesity-associated HCC development
The hypothesis of this project is that specific signalling pathways are dysregulated by obesity and inflammation in the liver. Oxidative stress caused by inflammation affects the activity of metabolic proteins, which could trigger the processes that cause hepatic dysfunction and HCC development. To test our hypothesis, we will develop tools that will allow us to identify major inactivated metabolic proteins and their roles in cellular responses in obesity-associated HCC.
We use an integrated analytical pipeline to determine the complete gene and protein expression changes between steatosis, MASLD, MASH, and HCC. We combine genetic analyses using single nuclei transcriptomic sequencing (sNuc-seq), spatial transcriptomics analysis using the Visium platform (10X Genomics), and a quantitative proteomics approach to identify transcriptional and post-transcriptional signatures during the transition from steatosis to HCC.
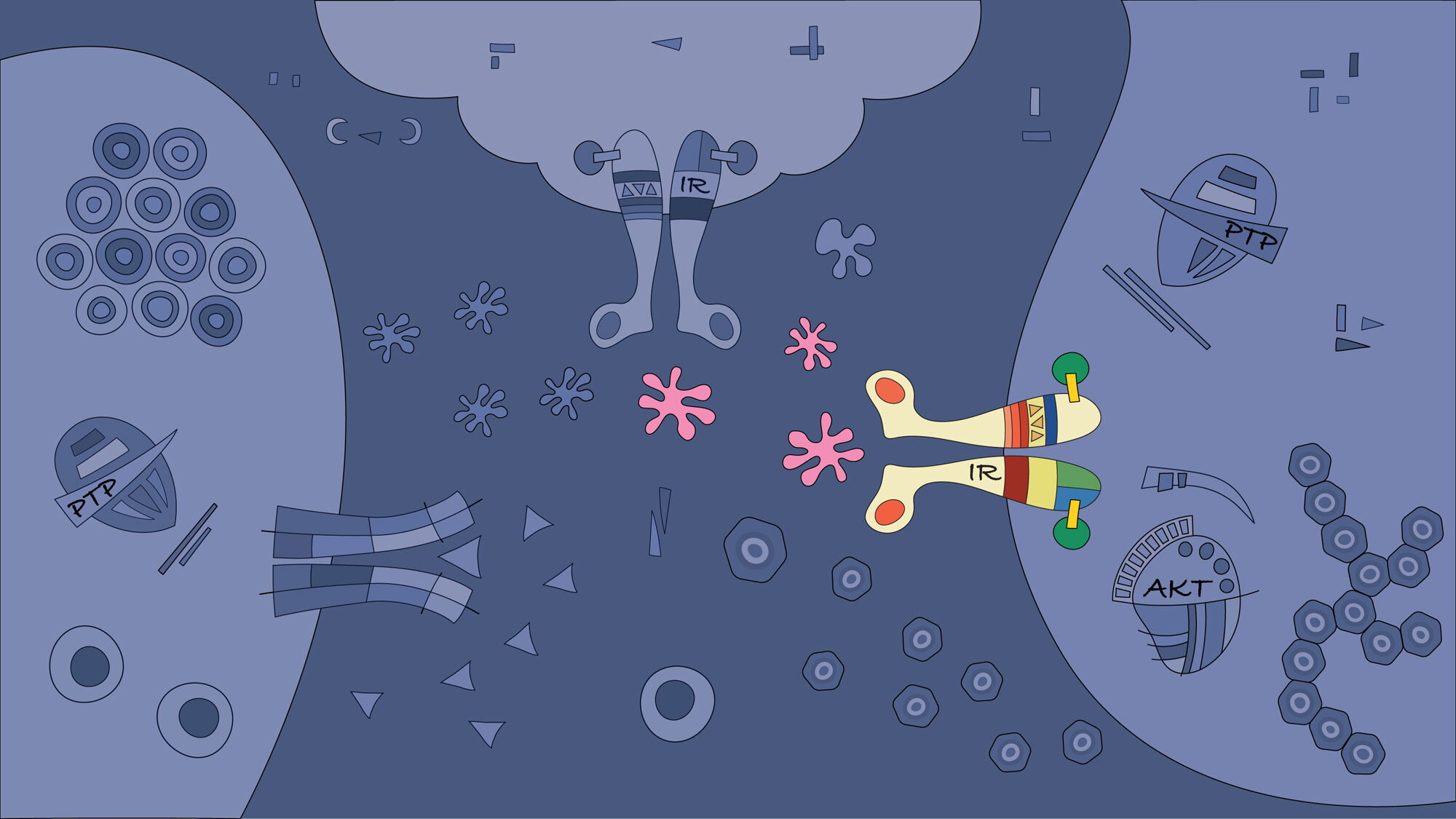
Based on our previous data and unpublished observations, we predict that research strategies based on a family of proteins known as tyrosine phosphatases will allow us to better understand the transition from fat accumulation in the hepatocytes to hepatic cancer development. This information is critical for new pharmacological screening. We aim to restore protein tyrosine phosphatase activity and associated dysfunctional signalling in patients. (Figure adapted from Brahma MK, Gilglioni EH et al. Oncogene 2021)
Selected Publications
Gilglioni EH, Li A, St-Pierre-Wijckmans W, Shen TK, Pérez-Chávez I, Hovhannisyan G, Lisjak M, Negueruela J, Vandenbempt V, Bauzá-Martinez J, Herranz JM, Ezerina E, Demine S, Feng Z, Vignane T, Otero-Sánchez L, Lambertucci F, Prašnická A, Devière J, Hay DC, Encinar JA, Singh SP, Messens J, Filipovic MR, Sharpe HJ, Trépo E, Wu W, Gurzov EN. Protein tyrosine phosphatase receptor kappa regulates glycolysis and de novo lipogenesis to promote hepatocyte metabolic reprogramming in obesity. Nature Communications, 15(01):9522, 2024
Pérez-Chávez I, Koberstein JN, Pueyo JM, Gilglioni EH, Vertommen D, Baeyens N, Ezeriņa D, Gurzov EN, Messens J. Tracking fructose 1,6-bisphosphate dynamics in liver cancer cells using a fluorescent biosensor. iScience 27(12):111336, 2024
Talamantes S, Lisjak M, Gilglioni EH, Llamoza-Torres CJ, Ramos-Molina B, Gurzov EN. Non-alcoholic fatty liver disease and diabetes mellitus as growing aetiologies of hepatocellular carcinoma. JHEP Rep 5(9):100811, 2023
Brahma MK, Gilglioni EH, Zhou L, Trepo E, Chen P, Gurzov EN. Oxidative Stress in Obesity-Associated Hepatocellular Carcinoma : Sources, Signaling and Therapeutic Challenges. Oncogene 40(33):5155-5167, 2021
Grohmann M, Wiede F, Dodd GT, Gurzov EN, Ooi GJ, Butt T, Rasmiena AA, Kaur S, Gulati T, Goh PK, Treloar AE, Archer S, Brown WA, Muller M, Watt MJ, Ohara O, McLean CA, Tiganis T. Obesity Drives STAT-1-Dependent NASH and STAT-3-Dependent HCC. Cell 175(5):1289-1306, 2018
Litwak SA, Pang L, Galic S, Igoillo-Esteve M, Stanley WJ, Turatsinze JV, Loh K, Thomas HE, Sharma A, Trepo E, Moreno C, Gough DJ, Eizirik DL, de Haan JB, Gurzov EN. JNK Activation of BIM Promotes Hepatic Oxidative Stress, Steatosis and Insulin Resistance in Obesity. Diabetes 66:2973-2986, 2017
Gurzov EN, Stanley WJ, Brodnicki TC, Thomas HE. Protein tyrosine phosphatases: molecular switches in metabolism and diabetes. Trends Endocrinol Metab 26(1):30-39, 2015
Gurzov EN, Tran M, Fernandez-Rojo M, Merry T, Zhang X, Xu Y, Fukushima A, Waters MJ, Watt M, Andrikopoulos S, Neel BG, Tiganis T. Hepatic oxidative stress promotes insulin-STAT5 signaling and obesity by inactivating PTPN2. Cell Metabolism 20(1):85-102, 2014
Support our research
You have the opportunity to become a supporter of the work being undertaken at the Signal Transduction & Metabolism Laboratory - your support will help the new generation of junior researchers become experienced scientists and may make a difference in the search of better treatments for diabetes and liver cancer.


